Clinical Trials Enrolment
89bio BIO89-100-131 (Metabolic Dysfunction-Associated Steatohepatitis)
Purpose of this Study
We are doing this study to find out if an experimental drug called pegozafermin (the study drug) is a safe and effective option for people with Metabolic Dysfunction Associated Steatohepatitis (MASH).
Who Can Participate?
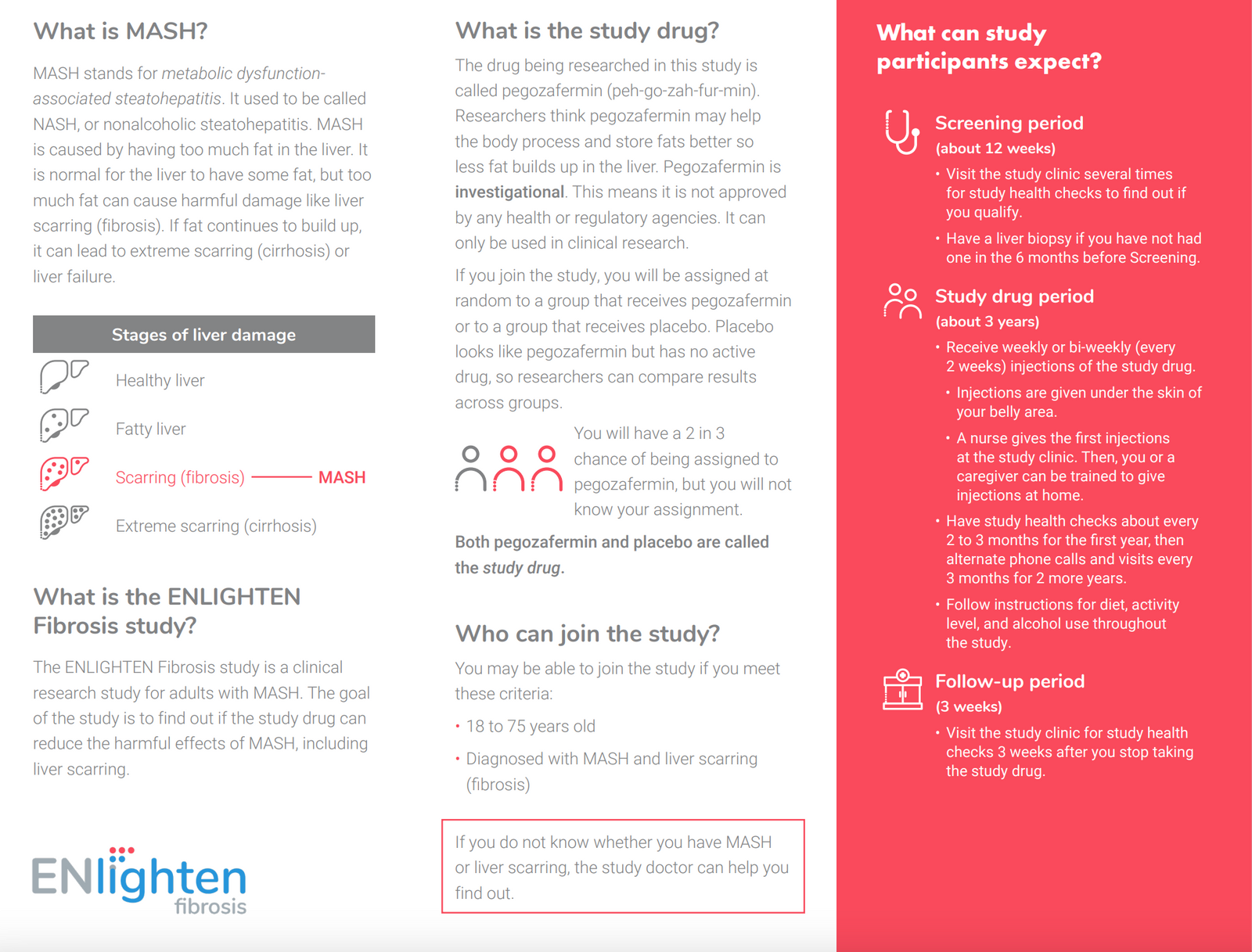
Eligibility
Adults ages 18-75 who:
- Are diagnosed with MASH confirmed by liver biopsy
- Have fibrosis stage F2 or F3
- Have at least 2 of the following risk factors: waist circumference >37 inches for males or >32 inches for females, body mass index >25 (>23 if Asian), diagnosis of Type 2 Diabetes, hypertriglyceridemia, HDL cholesterol <40 for males or <50 for females, hypertension
Age Range
18-75
Sex/Genders
Male (cisgender)
Female (cisgender)
Non-binary or gender fluid
Transgender male
Transgender female
A Phase 3 Study to Evaluate the Efficacy and Safety of Pegozafermin in Subjects with Compensated Cirrhosis due to Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Brief description of study
The purpose of this study is to learn about the safety, effectiveness, and long-term outcomes of the research drug, pegozafermin, for patients with compensated cirrhosis due to MASH when compared to a placebo.
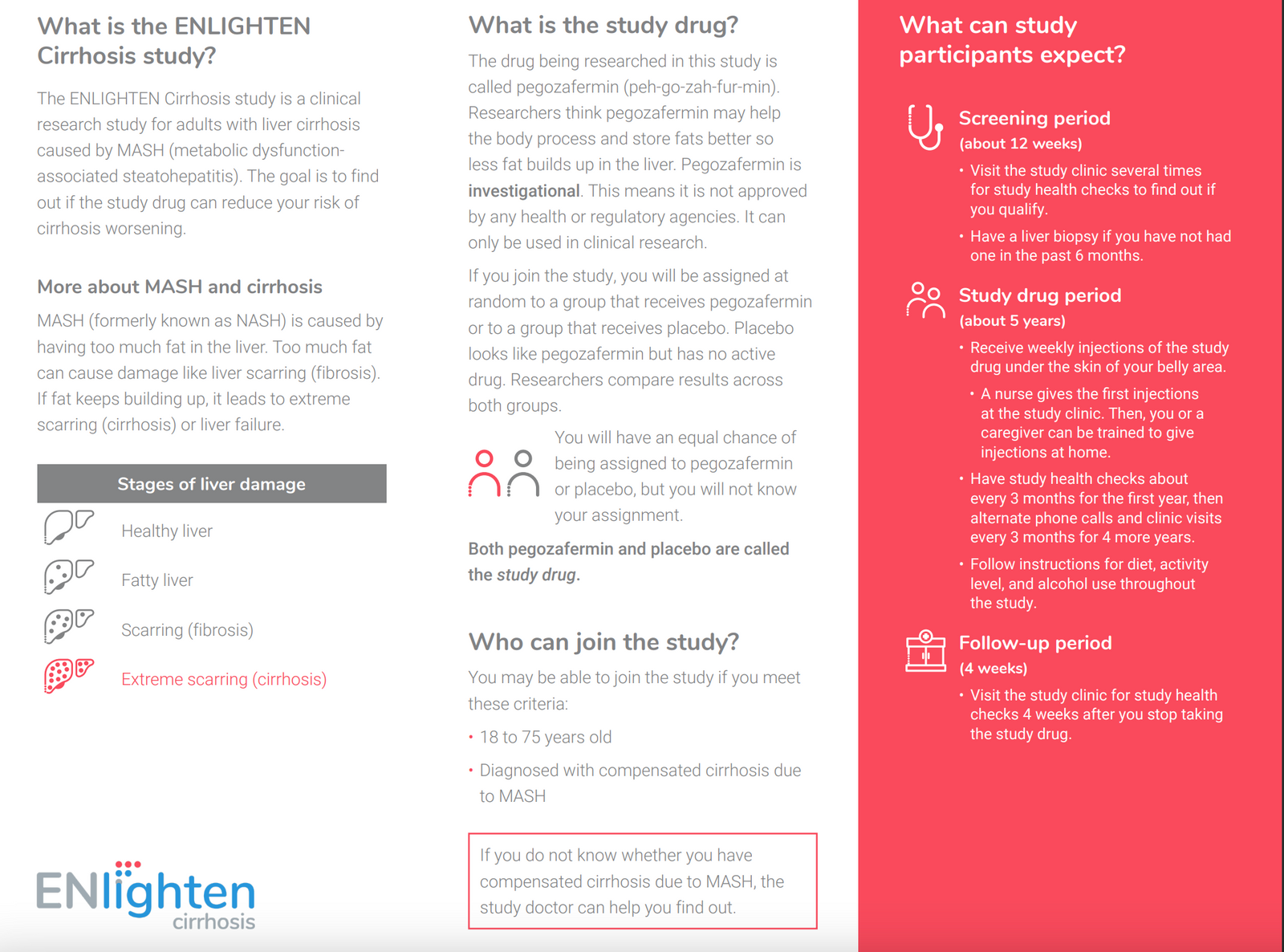
Are you eligible to participate in this study?
You may be eligible for this study if you meet the following criteria:
Conditions:
Metabolic Dysfunction-associated Steatohepatitis (MASH)
Age: Between 18 Year(s) - 75 Year(s)
Gender: Male or Female
Other Inclusion Criteria:
- Males or non-pregnant females aged between 18 and 75 years (inclusive) at the time of signing the informed consent form (ICF).
- Presence of at least one metabolic risk factor
- Evidence of both compensated cirrhosis (biopsy-confirmed fibrosis stage F4 per NASH CRN system) and MASH etiology (per modified Liver Forum Criteria as defined in protocol), either through a historical biopsy or a biopsy at Screening
- Body mass index (BMI) at Screening ≥25.0 (≥23.0 for Asian subjects) and <50.0 kg/m2
You may not be eligible for this study if the following are true:
- Chronic liver diseases other than MASH
- Past or current evidence of hepatic decompensation, including bleeding from gastroesophageal varices, cirrhotic ascites, or hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, or acute on chronic liver failure
- Past or current evidence of large esophageal or gastric varices, varices with “red-wale” signs, regardless of size on endoscopy
- Uncontrolled or newly diagnosed (≤3 months since diagnosis) type 2 diabetes
- Type 1 diabetes
- Child-Pugh Class B or C status
- Contact us if you think you might qualify.